LifePlus is on the verge of introducing a truly non-invasive continuous glucose monitoring wearable product to the market, one of the first of its kind. The company is committed to attain a very high-grade accuracy for the product before relasing it to the global market, and curently active in multi-site clincial trials across different demographies toward achieving this goal.
The Benefits of LifeLeafTM

Clinical Grade
LifeLeaf is in the process of attaining clinical grade accuracy through global multi-site clinical trials.

Non-Invasive
We use a non-invasive optical signal to measure blood glucose and other parameters.

Continuous
Measurements taken every few minutes capture continuous blood glucose and other trends.
Real-Time
Blood glucose levels are measured from blood vessels underneath the skin and reports in real time.
Intelligent
A powerful AI engine generates customizable and personalized alerts and insights.

Affordable
With no consumables, a significantly lower recurrent cost makes it highly affordable.
Secure
The data flow is secured with end-to-end encryption, hiding user identity making it fully HIPAA-compliant.
See LifeLeaf in Action
Introducing LifeLeaf


How LifeLeaf Works
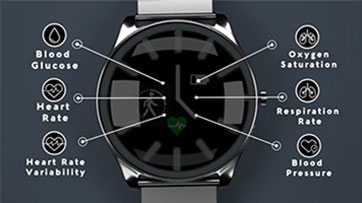

What Users Are Saying


Early Enrollment
LifePlus is accepting beta testers for their recently released LifeLeaf non-invasive physiological monitoring platform prototype. It includes a full functioning wearable smartwatch that continuously monitors major physiological parameters including blood glucose, blood pressure, heart rate etc, and is connected with a mobile application with backend cloud-based AI analytics.


















